The Beer-lambert Law Is a Linear Relationship for
The linear relationship between absorbance and concentration is very strong. While working in concentration units of molarity the Beers law is written as aecl.
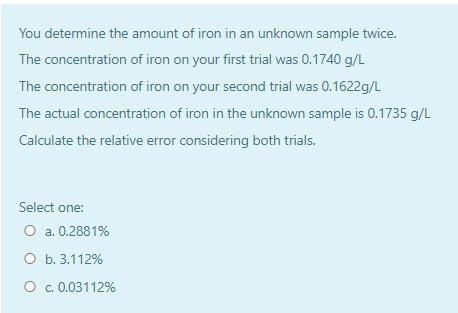
Solved The Beer Lambert Law Is A Linear Relationship For Chegg Com
Where a is the Absorbance e is the Molar.

. L o exp x µ s ρ b 3 where l is the detected photon count rate l 0 is the ra. From this it is clear that one would use absorbance as the scale for any UVVisNIR application that involved quantitation. A 2nd order polynomial relationship between absorption and concentration is sometimes encountered for very large complex molecules such as organic dyes Xylenol Orange or Neutral Red for.
From Literature to Law we have MA and PhD. It was discovered first in chemistry for optical photons ie. This law states that the absorbance of a light absorbing material is proportional to its concentration in solution.
Regular light but the same behavior applies for x-rays as well. All the papers we deliver to clients are based on credible sources and are quality-approved by our editors. Well just call it Beers Law for simplification in.
It is the linear relationship between absorbance and concentration of an absorbing species. According to the Beer-Lambert Law A εbc where ε is the molar absortivity with units Lmol-1cm-1 b is the length of the sample path length of the cuvette and c is concentration in mol L-1. We can write proofread paraphrase format edit or rewrite your any paper whether its a review or a term paper.
Lobsey and Viscarra Rossel 2016 as follows. The additional names come from different individuals who made contributions to the discovery of this basic physic behavior. The BeerLambert Law is useful for characterizing many compounds but does not hold as a universal relationship for the concentration and absorption of all substances.
The linear relationship between concentration and absorbance is both simple and straightforward which is why we prefer to express the Beer-Lambert law using absorbance as a measure of the absorption rather than T. Beers Law has several names including Beer-Lambert and BeerLambertBouguer law. Soil is defined by Beer-Lamberts law Smith 2000.
Gauss Law also known as Gausss flux theorem or Gausss theorem can be referred to as the law which explains the relation between electric charge distribution to the resulting electric field. Figure 2 shows the calibration curve for acetophenone. Experts in almost any academic discipline for any task.
This relationship is expressed by the Lambert-Beer law which is more commonly known as Beers law. Our writers can. With absorbance we can.
The Beer Lambert Law is also known as Beers law Beer-Lambert-Bouguer law or Lambert-Beer law. It is because of this relationship that biologists measure absorption rather than transmission. This law was discovered by Pierre Bouguer before 1729.
The electrical field of the surface is calculated by applying Coulombs law but to calculate the distribution of the electrical field on a closed surface the Gauss law is required.
Why Is The Relation Between Concentration And Absorption Linear Based On Beer S Law Quora

Solved The Beer Lambert Law Is A Linear Relationship For Chegg Com

No comments for "The Beer-lambert Law Is a Linear Relationship for"
Post a Comment